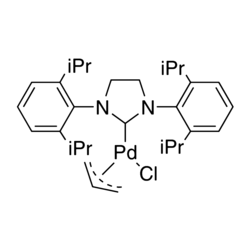
Pd(OH)2/C (Pearlman's Catalyst): A Highly Active Catalyst for Fukuyama, Sonogashira, and Suzuki Coupling Reactions | The Journal of Organic Chemistry

Direct arylation of heteroaromatic substrate with Pearlman's catalyst. | Download Scientific Diagram

Fukuyama reduction, Fukuyama coupling and Fukuyama–Mitsunobu alkylation: recent developments and synthetic applications | SpringerLink

Hydroxyl group deprotection reactions with Pd(OH)2/C: a convenient alternative to hydrogenolysis of benzyl ethers and acid hydrolysis of ketals | Semantic Scholar

Scheme 7 Hydrosilylation of aldehydes catalyzed by a nickel PCP-pincer... | Download Scientific Diagram

Pd/C: An Old Catalyst for New Applications – Its Use for the Suzuki–Miyaura Reaction - Felpin - 2006 - European Journal of Organic Chemistry - Wiley Online Library
![Elaboration of the ether cleaving ability and selectivity of the classical Pearlman's catalyst [Pd(OH)2/C]: concise synthesis of a precursor for a myo-inositol pyrophosphate - ScienceDirect Elaboration of the ether cleaving ability and selectivity of the classical Pearlman's catalyst [Pd(OH)2/C]: concise synthesis of a precursor for a myo-inositol pyrophosphate - ScienceDirect](https://ars.els-cdn.com/content/image/1-s2.0-S0040402012013944-sc4.jpg)
Elaboration of the ether cleaving ability and selectivity of the classical Pearlman's catalyst [Pd(OH)2/C]: concise synthesis of a precursor for a myo-inositol pyrophosphate - ScienceDirect

Pd(OH)2/C (Pearlman's Catalyst): A Highly Active Catalyst for Fukuyama, Sonogashira, and Suzuki Coupling Reactions | The Journal of Organic Chemistry

Direct arylation of heteroaromatic substrate with Pearlman's catalyst. | Download Scientific Diagram
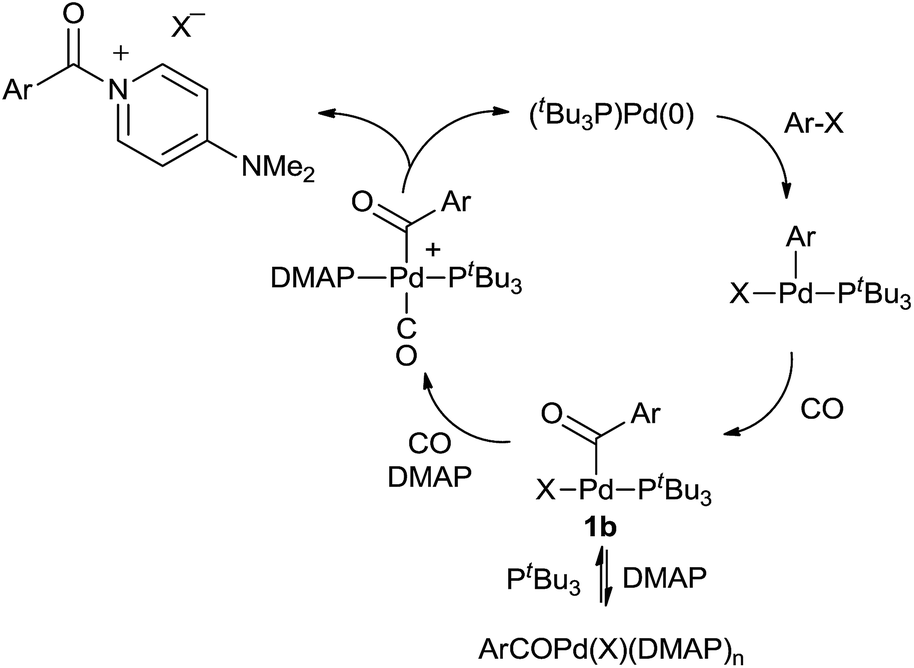
A flexible approach to Pd-catalyzed carbonylations via aroyl dimethylaminopyridinium salts - Chemical Science (RSC Publishing) DOI:10.1039/C5SC02949J

Pd(OH)2/C (Pearlman's Catalyst): A Highly Active Catalyst for Fukuyama, Sonogashira, and Suzuki Coupling Reactions | The Journal of Organic Chemistry
![Elaboration of the ether cleaving ability and selectivity of the classical Pearlman's catalyst [Pd(OH)2/C]: concise synthesis of a precursor for a myo-inositol pyrophosphate - ScienceDirect Elaboration of the ether cleaving ability and selectivity of the classical Pearlman's catalyst [Pd(OH)2/C]: concise synthesis of a precursor for a myo-inositol pyrophosphate - ScienceDirect](https://ars.els-cdn.com/content/image/1-s2.0-S0040402012013944-sc6.jpg)
Elaboration of the ether cleaving ability and selectivity of the classical Pearlman's catalyst [Pd(OH)2/C]: concise synthesis of a precursor for a myo-inositol pyrophosphate - ScienceDirect
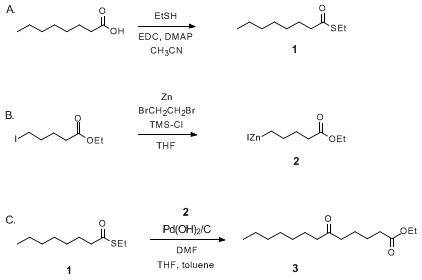
Pd(OH)2/C, a Practical and Efficient Catalyst for the Carboxylation of Benzylic Bromides with Carbon Monoxide
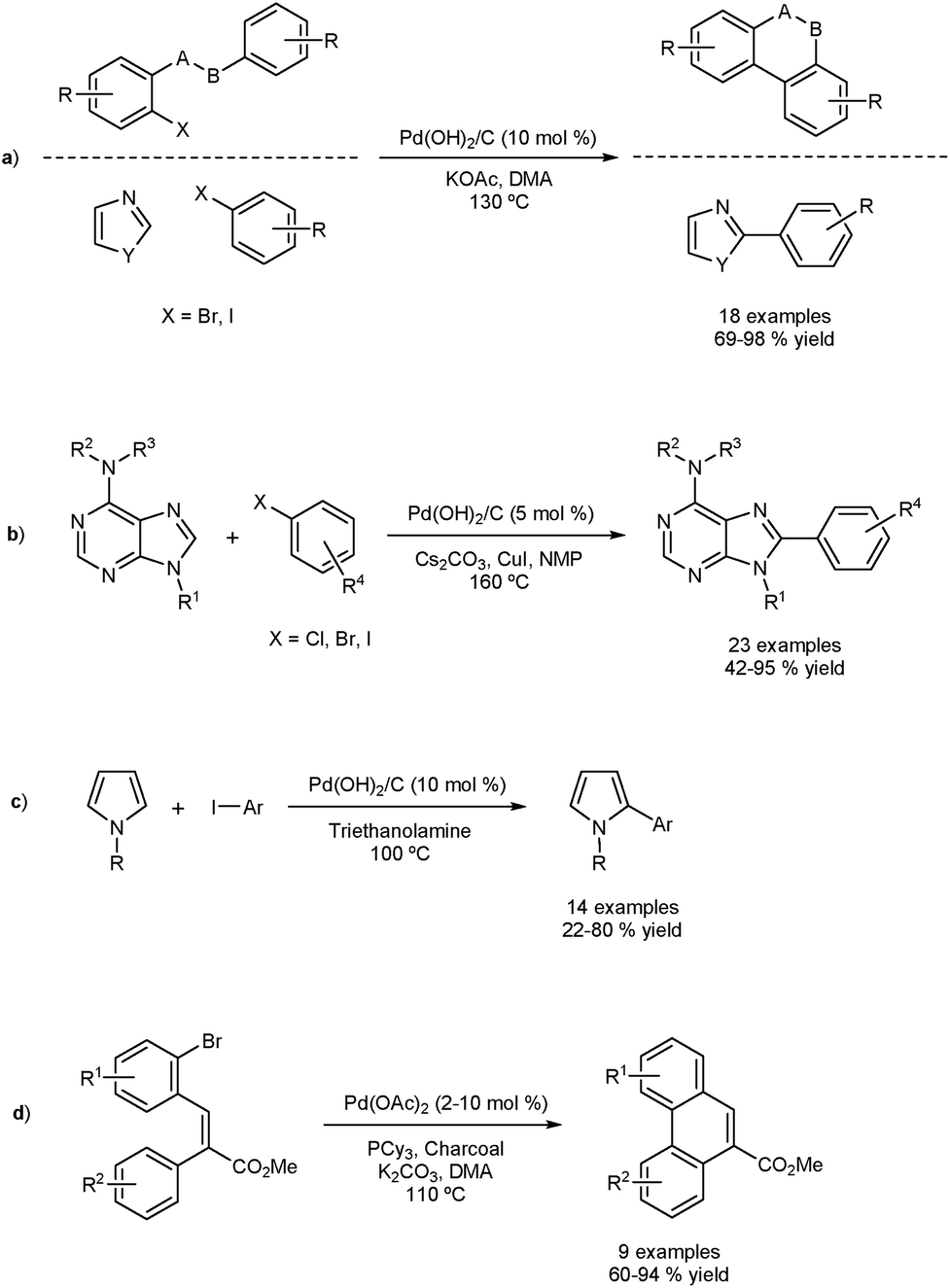
Direct arylation and heterogeneous catalysis; ever the twain shall meet - Chemical Science (RSC Publishing) DOI:10.1039/C5SC01534K
Pd(OH)2/C, a Practical and Efficient Catalyst for the Carboxylation of Benzylic Bromides with Carbon Monoxide
![Elaboration of the ether cleaving ability and selectivity of the classical Pearlman's catalyst [Pd(OH)2/C]: concise synthesis of a precursor for a myo-inositol pyrophosphate - ScienceDirect Elaboration of the ether cleaving ability and selectivity of the classical Pearlman's catalyst [Pd(OH)2/C]: concise synthesis of a precursor for a myo-inositol pyrophosphate - ScienceDirect](https://ars.els-cdn.com/content/image/1-s2.0-S0040402012013944-sc2.jpg)